The Tell Me Fast Novel Coronavirus COVID-19 IgGIgM Antibody Test is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM. Our COVID-19 IgGIgM Rapid Test Cassette is an Antibody test.
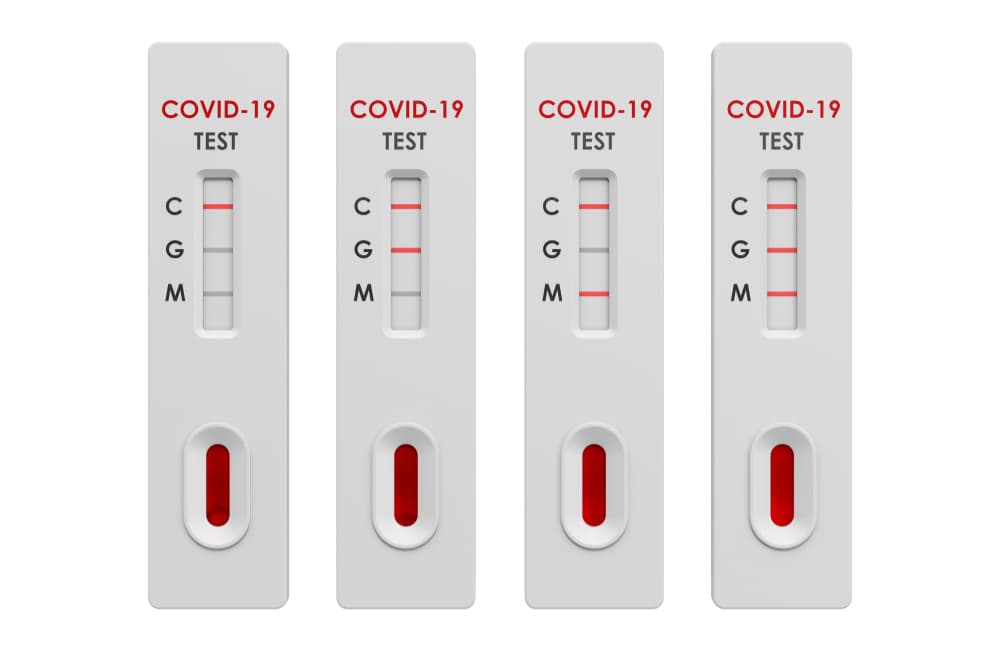 Sars Cov 2 Covid 19 Diagnosis By Igg Igm Rapid Test Clinisciences
Sars Cov 2 Covid 19 Diagnosis By Igg Igm Rapid Test Clinisciences
By Dalton Dunaway PharmD BCMAS.
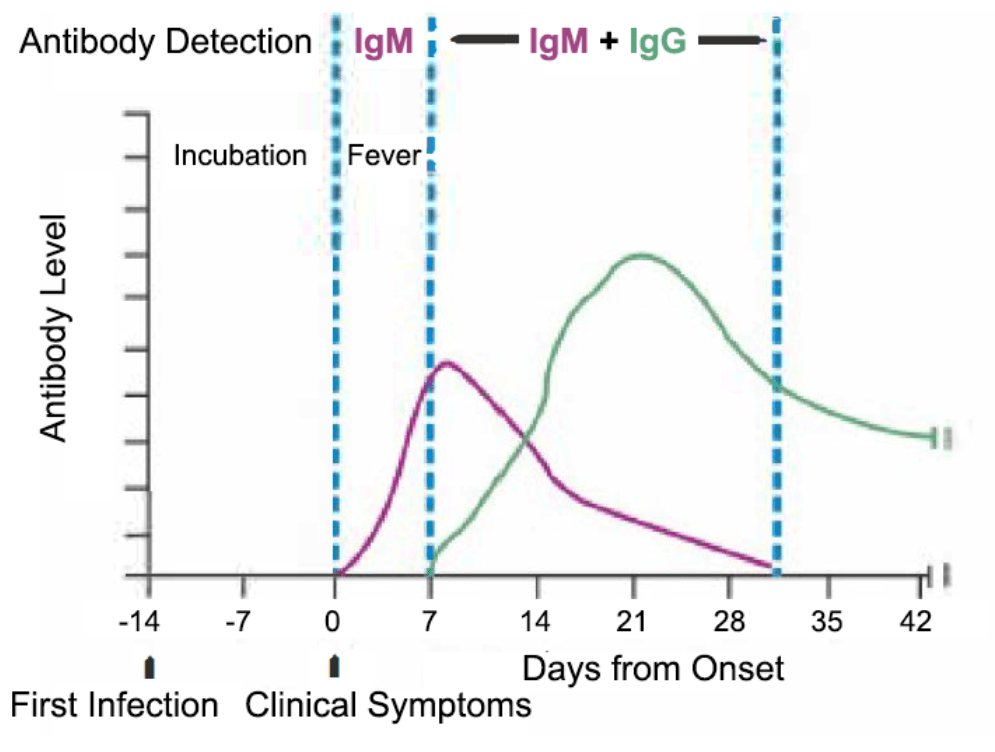
Igg and igm test. Antibodies are proteins made by the immune system to fight antigens such as bacteria viruses and toxins. Expert analysts and forecasters cite that COVID-19 IgMIgG Rapid Test Kits market size is set to grow at an annual rate of XX during the forecast period. An IgG test on the other hand helps determine the immunity status following a virus infection or active immunization or sometimes it helps diagnose a persistent infection.
IgG antibodies are the only type of antibody that can cross the placenta therefore the IgG antibodies of a pregnant woman. It also helps determine whether a patient has protection toward COVID-19. It is intended to confirm that a person has developed the antibodies that protect a person from getting a severe COVID-19 infection or hospitalization.
IgM antibodies are larger than IgG antibodies and when present in high numbers may indicate a recent or new active infection. An IgM test determines if someone has been recently infected or sometimes it determines if someone is still infected. There are an emerging amount of IgG and IgM antibody tests for COVID-19 that are being developed and presented to clinicians.
Doctors often measure IgA IgG and IgM together to get a snapshot of your immune function. Time the IgG western blot must be positive in order to indicate a potential infection with Lyme disease. The presence of IgG antibodies to an organism when accompanied by a negative IgM test for the same organism means that the person was exposed to that organism at one time and developed antibodies to it but does not have a current active infection of that organism.
COVID-19 IgGIgM Test Model. Spike Protein IgG Antibody Test This is a blood test. The body makes different immunoglobulins to combat different antigens.
This test is the most sensitive testing method and can identify the virus. Point-of-care POC tests generally are lateral flow devices that detect IgG and IgM or total antibody in fingerstick whole blood. It is designed to detect IgG antibodies specific for the virus spike protein that develop once a person has received the COVID-19 vaccination.
A lab tech will usually take a sample of your blood by inserting a. New The Humasis COVID-19 IgGIgM Test is an in vitro diagnostic test based on lateral flow chromatographic immunoassay intended for qualitative detection of IgG and IgM antibodies specific to SARS-CoV-2 in human whole blood plasma or serum. In short a positive IgM may be a sign of a current or very recent infection.
An immunoglobulin test measures the level of certain immunoglobulins or antibodies in the blood. IgG starts spiking as IgM starts coming down. It detects the coronavirus antibodies that are in the bloodstream after people have become infected.
A positive test result with the qSARS-CoV-2 IgGIgM Rapid Test indicates that antibodies to SARS -CoV 2 were detected and the patient has potentially been-19. Humasis COVID-19 IgGIgM Test Condition. IgG antibodies are important for fighting bacterial and viral infections.
This test should not be used with heat inactivated or other inactivated human specimen blood serum plasma. When it comes to Borrelia. BioMedomics Rapid IgM-IgG Combined Antibody Test for COVID-19 is a lateral flow immunoassay used to qualitatively detect IgG and IgM antibodies of the novel coronavirus in human serum plasma or whole blood in vitro.
The IgM antibodies are the first antibodies to be produced in the body in response to an infection. The COVID-19 IgGIgM Rapid Test detects IgM and IgG antibodies in a rapid test that gives results within 2 to 10 minutes. Currently the most widely used method for diagnosing COVID-19 is the standard M PCR.
IgM and IgG Antibody Tests for COVID-19. Apr 15 2021 The Expresswire -- Final Report will add the analysis of the impact of COVID-19 on this industry Global COVID-19 IgMIgG Rapid Test Kits. The business intelligence report on COVID-19 IgMIgG Rapid Test Kits Market hosts latest industry data and projections supported by historical statistics and growth opportunities linked to the industry expansion over 2021-2026.
Laboratory tests use lateral flow ELISA or chemiluminescent immunoassay CIA methods for antibody detection in serum plasma whole blood and dried blood spots which for some assays may require trained laboratorians and specialized. Immunoglobulins IgA IgG IgM What It Is. The test which is user friendly has the COVID-19 IgGIgM Rapid Test Device capillary kit which is a qualitative membrane-based immunoassay for the detection of COVID-19 antibodies in whole blood serum or plasma.
Selfdiagnostics Sars Cov 2 Igm Igg Antibody Assay Kit Selfdiagnostics
 Qsars Cov 2 Igg Igm Rapid Test A Sages Technology And Value Assessment
Qsars Cov 2 Igg Igm Rapid Test A Sages Technology And Value Assessment
 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
 Vazyme 2019 Ncov Igg Igm Detection Kit Colloidal Gold Based Assay Genie
Vazyme 2019 Ncov Igg Igm Detection Kit Colloidal Gold Based Assay Genie
Nano Check Tm Covid 19 Igg Igm Antibody Test Nano Ditech
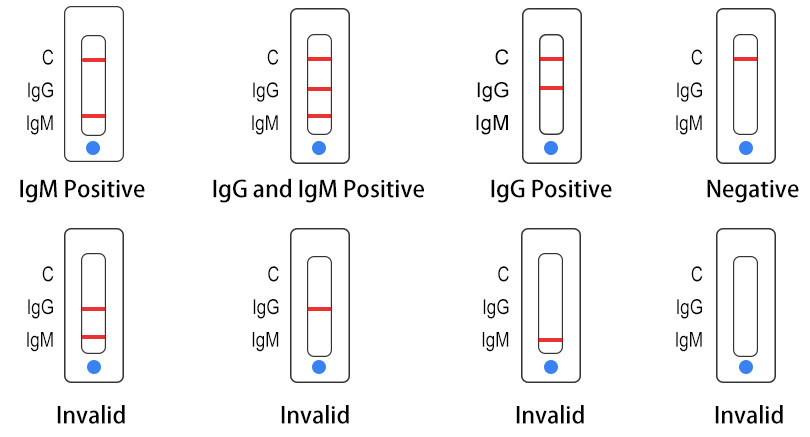 Uncov 40 Covid 19 Igm Igg Rapid Test In Stock In Paris 24 48
Uncov 40 Covid 19 Igm Igg Rapid Test In Stock In Paris 24 48
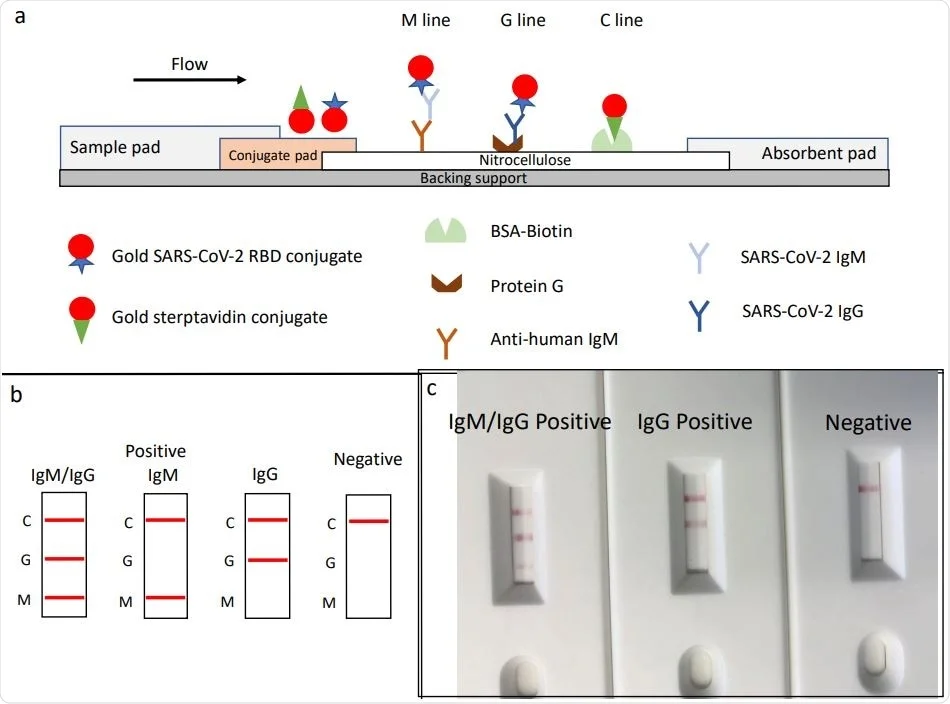 Rapid Sars Cov 2 Igm Igg Combined Antibody Test
Rapid Sars Cov 2 Igm Igg Combined Antibody Test
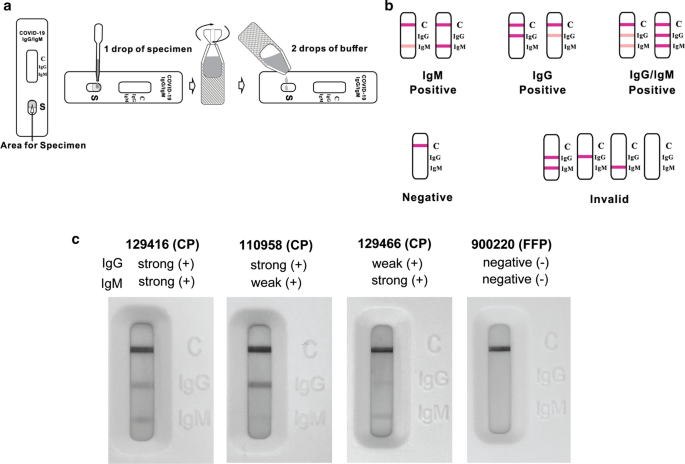 Covid19 Antibody Detection Using Lateral Flow Assay Tests In A Cohort Of Convalescent Plasma Donors Bmc Research Notes Full Text
Covid19 Antibody Detection Using Lateral Flow Assay Tests In A Cohort Of Convalescent Plasma Donors Bmc Research Notes Full Text
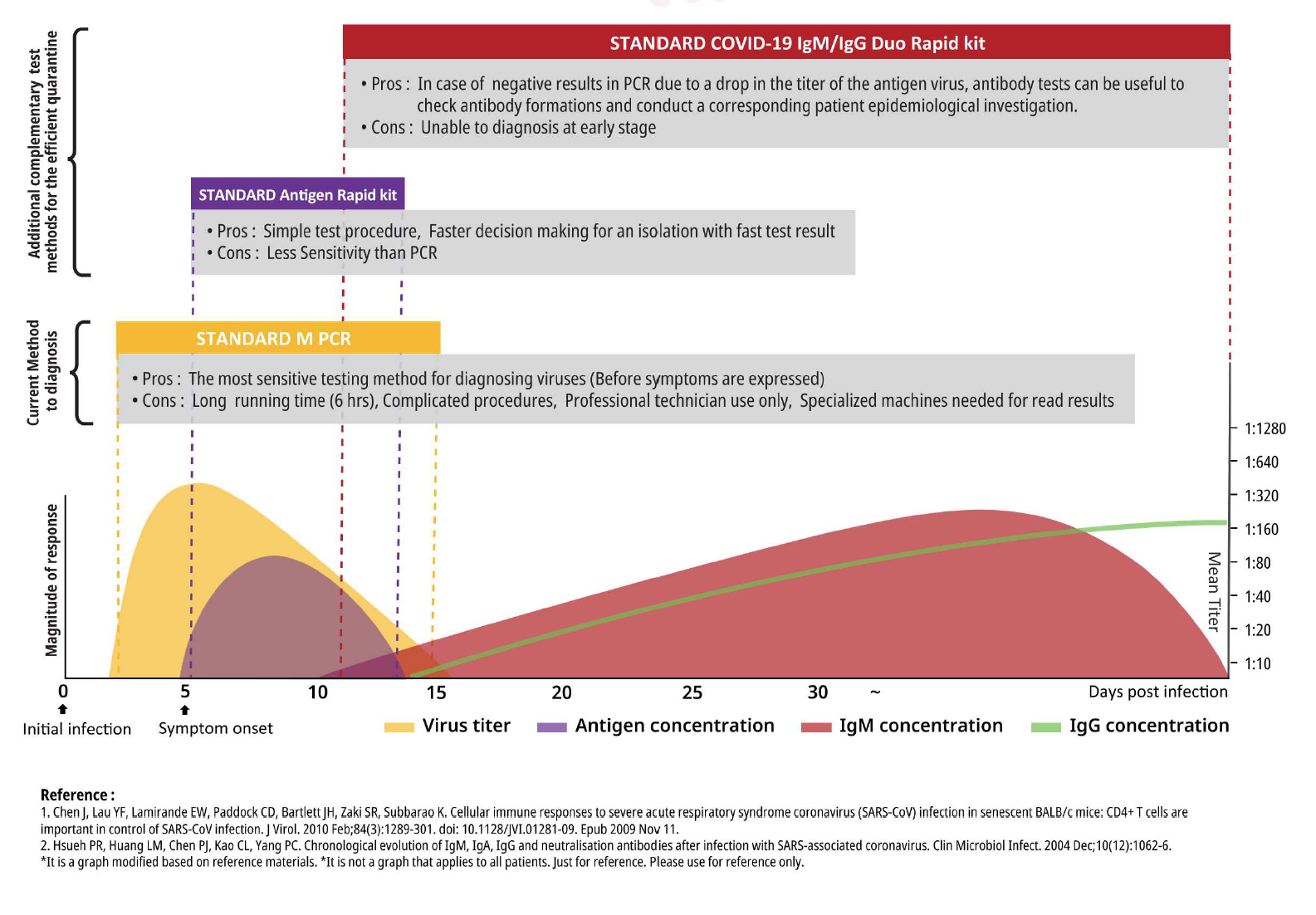 Igm And Igg Antibody Tests For Covid 19 Mdvip
Igm And Igg Antibody Tests For Covid 19 Mdvip
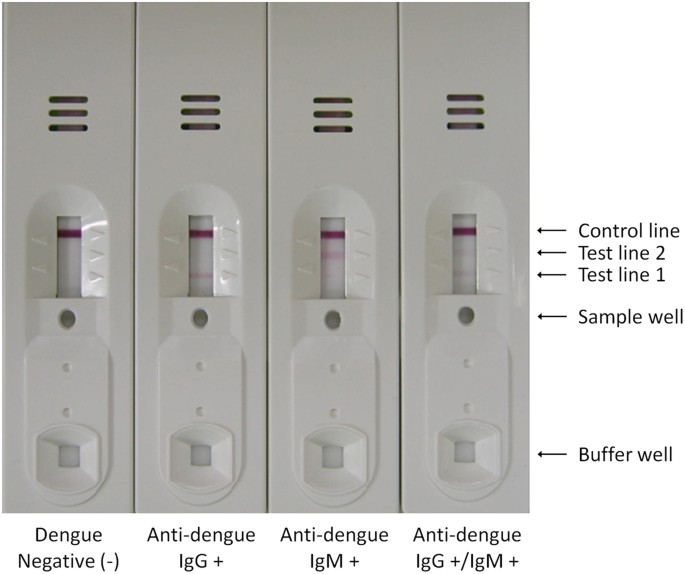 Enhanced Performance Of An Innovative Dengue Igg Igm Rapid Diagnostic Test Using An Anti Dengue Edi Monoclonal Antibody And Dengue Virus Antigen Scientific Reports
Enhanced Performance Of An Innovative Dengue Igg Igm Rapid Diagnostic Test Using An Anti Dengue Edi Monoclonal Antibody And Dengue Virus Antigen Scientific Reports
 Evolved Value Proposition Of The Onsite Covid 19 Igg Igm Rapid Test Ctk Biotech
Evolved Value Proposition Of The Onsite Covid 19 Igg Igm Rapid Test Ctk Biotech
 Covid 19 Igg Igm Rapid Test Prima Home Test
Covid 19 Igg Igm Rapid Test Prima Home Test
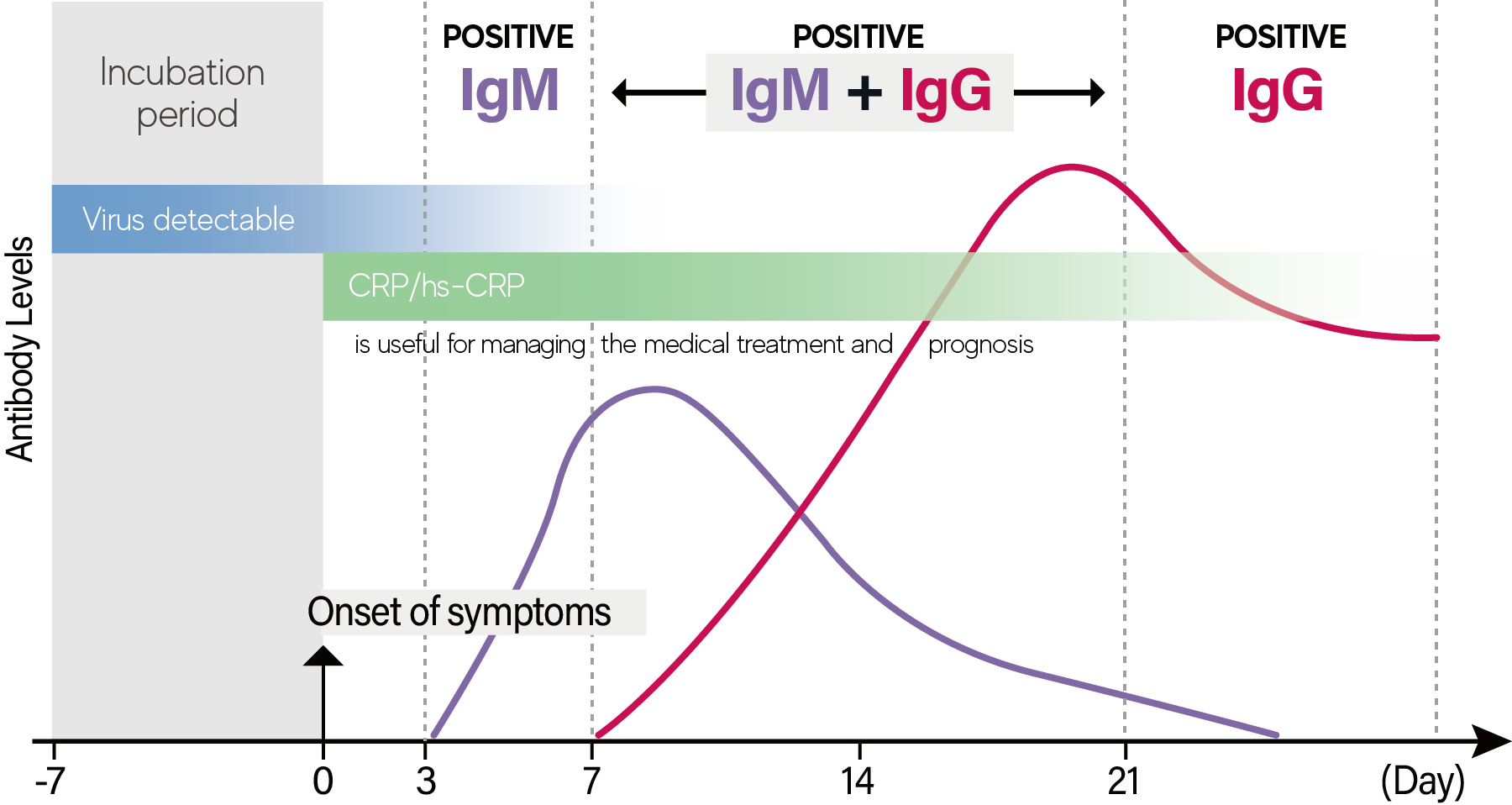


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.