Sodium hydroxide - the most common base in the world. The concentration of sodium hydroxide in water can be easily and quickly determined by refractive index measurement.
 Effect Of Concentration Of Sodium Hydroxide Solution On Sio 2 Download Table
Effect Of Concentration Of Sodium Hydroxide Solution On Sio 2 Download Table
The table below gives the density kgL and the corresponding concentration weight of Sodium Hydroxide in water at.

Concentration of sodium hydroxide. The most common concentration of NaOH is 50 although it can range from trace to about 70. The alkali will now have a concentration of 0. An experimental study was conducted to investigate the effects of erythropoietin on the acute phase of esophageal burn damage induced by sodium hydroxide.
Has made available its data on the ability of sodium hydroxide to inactivate eight different viruses. 01 M sodium hydroxide was sufficient to inactivate the murine leukemia virus a commonly used model enveloped virus 6. A standard esophageal alkaline burn was produced by the application of 10 sodium hydroxide to.
Sodium hydroxide water soluble solid white in colour a strong alkaliUsually in the form of lumps stickschips or pellets that upon solution in water generates heat. Sodium hydroxide NaOH caustic soda lye is a strong alkali base. Use the Standardized NaOH Solution to Determine the Concentration of an Acid.
Calculate the concentration of the sodium hydroxide solutionThe rest of the sodium hydroxide solution can now be used in further lab work as a secondary standard with a reliably known concentration equal to the average of the three titrations. Sodium hydroxide is a manufactured substance and sodium hydroxide Na OH is sold as a solid cast flakes pearls compounders or as solutions with varying concentrations. The table was taken from the chemical engineering handbook.
50 ww Sodium hydroxide means that 100 g of solution contains 50 g of NaOH. It is most commonly found in the home as drain and oven cleaners in concentrations between 05 and 54. Viewed 500 times 0 begingroup The specific gravity of a 50 aqueous solution ceNaOH is pu15298 g cm-3 To my.
It is used as flakes granules or as an aqueous solution mostly at concentrations of 48 to 50. Winemakers usually buy sodium hydroxide solution of a known concentration usually 01 Normal. Active 7 months ago.
Prolonged or repeated skin contact may cause dermatitis. Potassium hydroxide and their mixtures with a concentration range of approximately 2. This reagent is relatively unstable and its concentration changes over time.
5 to 12 normal at temperatures of 25 35 45 55 and 65 c are carefully investigated. Contact with very high concentrations of sodium hydroxide can cause severe burns to the eyes skin digestive system or lungs resulting in permanent damage or death. Both the solid and liquid forms can cause severe injury to all tissues.
What is the concentration of sodium hydroxide. Sodium hydroxide also termed lye caustic soda and sodium hydrate is a white solid that dissolves in water to produce a strongly alkaline solution. Input a temperature and density within the range of the table to calculate for concentration or input concentration to calculate for density.
In a wine laboratory analyzing wine for TA VA and S02 involves the use of a sodium hydroxide NaOH reagent. At ambient temperature and up to 70 concentration 316 SS has an A rating and can be used for the. Both 01 M and 05 M sodium hydroxide.
The density of 50 ww Sodium hydroxide is 1515 gml at 25C which means that the weight of the 1 ml of Sodium hydroxide solution is 122 g at 25C. Density of a sodium hydroxide solution. Ask Question Asked 7 months ago.
Using a 25cm3 pipette washed out with some of the NaOH solution transfer 25cm3 of the solution to a 250 cm3 volumetric flask and fill to the line with distilled water. Concentration of Sodium hydroxide sodium hydroxide. To ensure the accuracy of analytical results it is important to periodically check the.
The calculator does automatic interpolation calculation for density or concentration values that are between those in the table. However non-SI units are still used in many countries. Convert between mass and molar concentration In System International SI units the concentration of compounds is measured in moles and mole-derived units per liter and liter-derived units.
Repeated inhalation of sodium hydroxide vapor can lead to. This is a table of density kgL and the corresponding concentration weight of Sodium Hydroxide in water at a temperature of 20 degrees centigrade. The most important industrial concentration is 50.
More recently QOne Biotech Ltd. Mostly at concentration of 48 to 50 of NaOH is used in many industries.
/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png) How To Prepare A Sodium Hydroxide Or Naoh Solution
How To Prepare A Sodium Hydroxide Or Naoh Solution
 Effect Of Stirring Time X 1 Sodium Hydroxide Concentration X 2 Download Table
Effect Of Stirring Time X 1 Sodium Hydroxide Concentration X 2 Download Table
 Moles And Solutions Specifications Moles And Solutions Calculate The Amount Of Substance In Moles Using Solution Volume And Concentration Ppt Download
Moles And Solutions Specifications Moles And Solutions Calculate The Amount Of Substance In Moles Using Solution Volume And Concentration Ppt Download
 Effect Of Sodium Hydroxide Concentration On Various Properties Of Geopolymer Concrete Semantic Scholar
Effect Of Sodium Hydroxide Concentration On Various Properties Of Geopolymer Concrete Semantic Scholar
 Ph Calculations And More In Fundamentals Of Pharmaceutics Sodium Hydroxide As A Buffer In Solution
Ph Calculations And More In Fundamentals Of Pharmaceutics Sodium Hydroxide As A Buffer In Solution
 Question Video Calculating Hydrochloric Acid Concentration From Titration With Sodium Hydroxide Nagwa
Question Video Calculating Hydrochloric Acid Concentration From Titration With Sodium Hydroxide Nagwa
 Effect Of Sodium Hydroxide Concentration On The Absorbance Of The Download Scientific Diagram
Effect Of Sodium Hydroxide Concentration On The Absorbance Of The Download Scientific Diagram
 Calculate The Concentration Of Naoh Solution G Ml Which Has The Youtube
Calculate The Concentration Of Naoh Solution G Ml Which Has The Youtube
 Effect Of Concentration Of Sodium Hydroxide Solution On Compressive Download Scientific Diagram
Effect Of Concentration Of Sodium Hydroxide Solution On Compressive Download Scientific Diagram
 Concentration Of Sodium Hydroxide Solutions Used For Alkali Wrapping Download Table
Concentration Of Sodium Hydroxide Solutions Used For Alkali Wrapping Download Table
 Titration Practical And Calculation Naoh And Hcl Youtube
Titration Practical And Calculation Naoh And Hcl Youtube
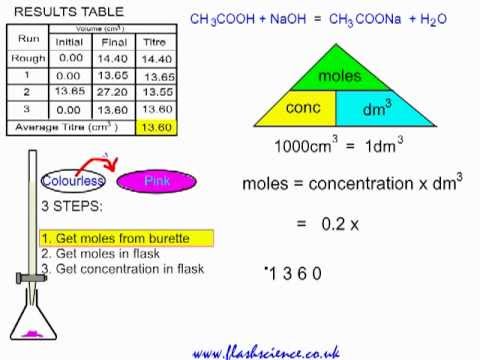 Titration Practical And Calculation Ethanoic Acid And Sodium Hydroxide Youtube
Titration Practical And Calculation Ethanoic Acid And Sodium Hydroxide Youtube
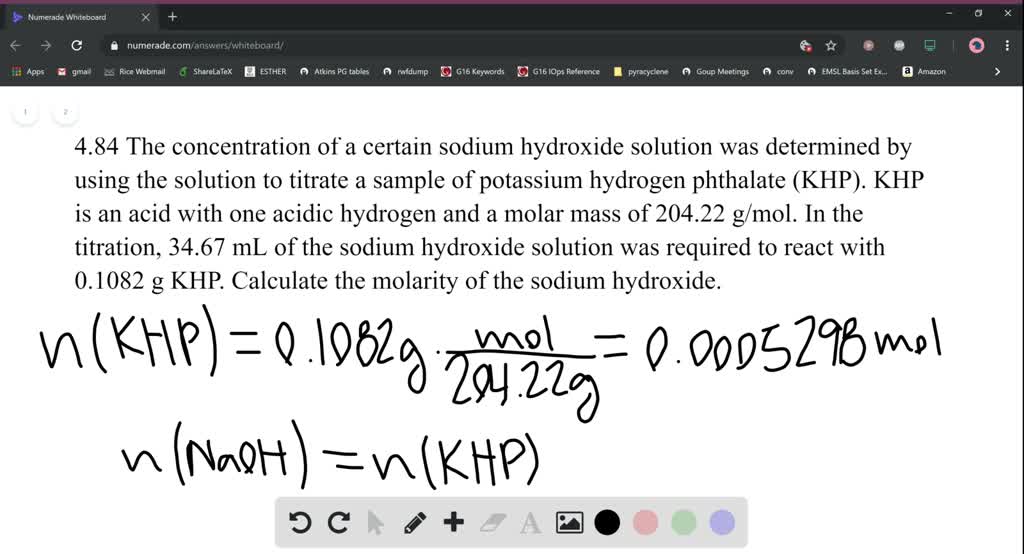 Solved The Concentration Of A Certain Sodium Hydr
Solved The Concentration Of A Certain Sodium Hydr
 The Effect Of Sodium Hydroxide Concentration On The Enzymatic Download Scientific Diagram
The Effect Of Sodium Hydroxide Concentration On The Enzymatic Download Scientific Diagram

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.