The FDAs decision to approve the expanded use for the cobas HPV Test was based on results from the landmark ATHENA trial which enrolled more than 47000 women. Both the intra- and interlaboratory agreement for the cobas HPV test were 98.
 Roche Cobas Hpv Test On Behance
Roche Cobas Hpv Test On Behance
Two cell-free supernatant fluid specimens yielded indeterminate cobas results.

Cobas hpv test. Cobas HPV testing had higher positive rates for the diagnosis of benign lesions 84 vs 51 and low-grade squamous intraepithelial lesions 89 vs 63 on biopsy compared with Aptima. The cobas HPV Test provides both pooled high-risk HPV DNA results and individual detection of HPV 16 and HPV 18 the two types responsible for about 70 percent of cervical cancer. 462464 biobanked samples from women with CIN3 tested HPV-positive on the cobas 6800 system corresponding to a relative sensitivity of 996.
Tewari D 1 Novak-Weekley S 2 Hong C 1 Aslam S 3 Behrens CM 3. The test manufactured by Roche Molecular Systems of Pleasanton CA is the same HPV test cobas HPV Test given FDA approval in 2011. The test utilizes 80 amplification of target DNA by the Polymerase Chain.
A valid cobas 4800 HPV test result was obtained for all samples Table 1. Twenty-three samples 56 were found to be p16 positive by immunohistochemistry. Detailed cobas 4800 HPV test results are given in Table 2.
The cobas test specifically identifies HRHPV types 16 and 18 while simultaneously detecting 12 other HRHPV types HPV31 HPV33 HPV35 HPV39 HPV45 HPV51 HPV52 HPV56 HPV58 HPV59 HPV66 and HPV68. Two of 20 Hybrid Capture 2-positiveRoche cobas-negative cases 10 and 26 of 37 Roche cobas-positiveHybrid Capture 2-negative cases 70 tested positive for high-risk human papillomavirus by Linear Array. Twenty-two samples were found to be positive on cobas hrHPV testing from both cell pellet and cell-free supernatant fluid.
Performance of the cobas HPV Test for the Triage of Atypical Squamous Cells of Undetermined Significance Cytology in Cervical Specimens Collected in SurePath. Both assays showed good agreement and excellent specificity with either ThinPrep or SurePath preparations. The test utilizes amplification of target DNA by the Polymerase Chain Reaction PCR and nucleic acid hybridization for the detection of 14 high-risk HPV types in a single analysis.
Of the cases 54 90 had a positive cobas 4800 HPV test result. The cobas HPV test is the first clinically validated FDA-approved CE-IVD marked HPV DNA test for primary cervical cancer screening ASC-US triage and co-testing. Of the controls the cobas 4800 HPV test gave a positive hrHPV result for 43 54 samples.
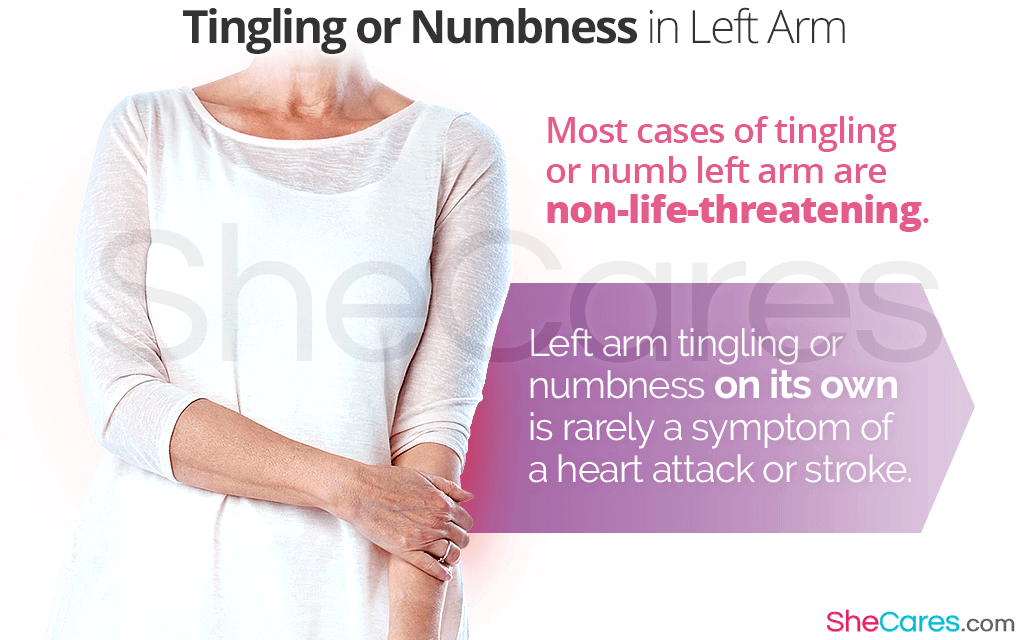
Aptima testing had significantly higher specificity for HSIL than Cobas 41 vs 13. On April 24 2014 the FDA approved the cobas HPV test as the first HPV DNA-based test for primary cervical cancer screening. 79 The cobas HPV Test is a qualitative in vitro test for the detection of Human Papillomavirus HPV in patient specimens.
Food and Drug Administration today approved the Roche cobas HPV Test as the first test for Human Papilloma Virus HPV that can be used with cervical cells obtained for a Pap test and. There was high reproducibility between the original cobas 4800 HPV test results and the cobas 4800 HPV re-testing performed on the samples retrieved from biobank storage. The cobas 4800 Human Papillomavirus HPV Test is a qualitative in vitro test for the detection of Human Papillomavirus in patient specimens.
The cobas HPV Test is a qualitative in vitro test for the detection of Human Papillomavirus in cervical specimens collected by a clinician using an endocervical brushspatula and placed in the. Persistent high-risk HPV infections can develop into precancerous lesions and if. The cobas HPV test provides pooled results on known high-risk HPV genotypes and simultaneous individual results from the two highest-risk HPV genotypes.
The FDA approved the cobas HPV Test as a first-line screening tool for cervical cancer in women aged 25 years and older in 2014 based on the results of the large ATHENA study which included specimens from 41955 women. HPV 16 and HPV 18 all from a. The cobas HPV Test is a qualitative in vitro test for the detection of Human Papillomavirus in cervical specimens collected by a clinician using an endocervical brushspatula and placed in the ThinPrep Pap Test PreservCyt Solution or using a cervical broom and placed in SurePath Preservative Fluid.
925932 biobanked samples from control women tested HPV-negative on. The cobas HPV test identifies women at risk for cervical cancer by detecting the presence of high-risk human papillomavirus HPV DNA in cervical samples. The cobas HPV test detected over 98 of histologically confirmed cervical intraepithelial neoplasia grade 2 CIN2 lesions in women age 30 years or older with a specificity of 989 compared with the reference cobas 4800 test.